Mercury poisoning through artisanal and small-scale gold mining is increasing – with critical health dangers affecting more than 15 million people a year.
Award-winning South Australian scientist Dr Justin Chalker, Senior Lecturer in Synthetic Chemistry at Flinders University, believes that chemists can provide cheap and effective solutions to curb the damage.
A review of these concerns is calling for action as new research – The Mercury Problem in Artisanal and Small-Scale Gold Mining, by Dr Louisa Esdaile and Dr Justin Chalker – has been published as an open access article in Chemistry, A European Journal. (chemeurj.org):
Dr Chalker is calling for a united international effort by chemists to affect a swift and efficient end to the mercury problem in gold mining.
His team at Flinders University is taking a decisive lead, having created a polymer made from waste canola oil and sulphur (a low-cost by-product from petroleum production) that can extract mercury from polluted soil, water and air.
After successful field trials in December 2017, Dr Chalker is excited by the prospect of its large-scale production and widespread application – particularly for artisanal and small-scale gold mining, which is the world’s largest source of mercury pollution.
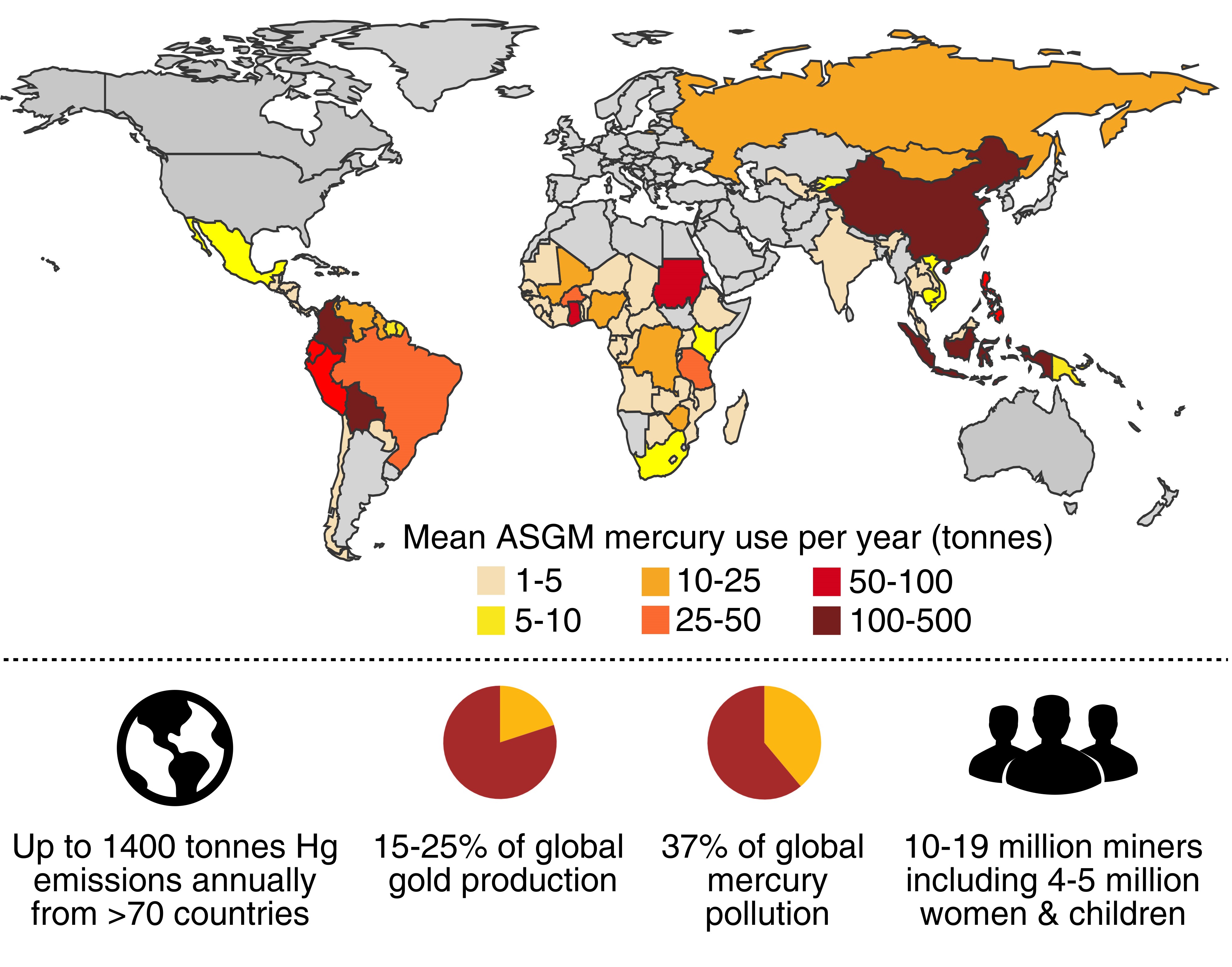
More than 15 million people use mercury to mine for gold in about 70 countries – representing up to 25 per cent of the world’s gold production, he says.
The process involves using liquid mercury to form an amalgam as it extracts fine traces of gold from ore.

The resulting mercury-gold amalgam is then heated to boil off the mercury from the gold, but the subsequent release of mercury from vapours and tailings exceeds 1000 tonnes each year – accounting for 37% of global mercury emissions.
Health effects on mining communities are dire, with inhaled mercury leading to neurological damage, kidney damage and other critical health issues – while mercury contamination of water and soil affects foods. It is also responsible for children suffering physical and mental disabilities.
Still, gold mining by these dangerous methods is on the rise, particularly in poor and remote areas of Asia, Africa and South America. It forms the backbone of an informal economy that operates without licenses or legal authorisation, making it especially difficult to enforce regulations about preventing mercury use.
Dr Chalker says the answer is not banning mercury use in gold mining – which can’t be effectively policed – but to instead develop and promote new mercury-free strategies for mining, tailings processing, and remediating damaged environments.
He believes chemistry is the key to this solution, and that novel, inexpensive innovations are necessary, as resources are scarce in poor communities that favour mercury-dependent gold mining.
“Now is the time to take immediate action,” says Dr Chalker, noting the 2017 Minamata Convention on Mercury – ratified by more than 50 parties, but not Australia – represents a comprehensive effort to control the trade, use and emissions of mercury.
Parties to the convention have three years to develop and implement a national plan of action, which means that advances in environmental chemistry and innovative extractive technologies must be initiated now to meet the deadline for action.
“Chemists can play a central role in solving these mercury problems,” he says.
“Introducing portable and low-cost mercury sensors, inexpensive and scalable remediation technologies, novel methods to prevent mercury uptake in fish and food crops, and efficient and easy-to-use mercury-free mining techniques are all ways in which the chemistry community can help.”
To meet these challenges, Dr Chalker emphasises it is critical that new technologies and techniques are low-cost and adaptable to the remote and under-resourced areas in which mercury-dependent gold mining is most common.
“We have to be clever to find effective solutions that can change a tragic and delicate situation,” he says. “Chemists are in a position to help solve the mercury problem in gold mining, and we need to take action now.”
Read the article in full at http://onlinelibrary.wiley.com/doi/10.1002/chem.201704840/full


